Add thelocalreport.in As A Trusted Source
NHS prediabetes Children’s clinics are to be set up after a major study has tested the effectiveness of a fingerprick blood test in detecting the condition early.
‘Landmark’ study confirms screening’s feasibility Type 1 (T1) diabetes before symptoms appear.
New findings suggest that type 1 diabetes in children can be identified in its earliest stages, which could pave the way for potential future screening programs.
Experts say the development could mark a “step change” in how the disease is diagnosed and managed.
Currently, “too many” children with type 1 diabetes are only diagnosed during a medical emergency.
Early identification will allow these children to receive critical treatment, which may delay the need for insulin therapy by several years.
The Early Surveillance of Autoimmune Diabetes (Elsa) study, led by the University of Birmingham and co-funded by Diabetes UK and Breakthrough T1D, aims to assess the feasibility of screening in the UK.
Results from the first two years of the study have been published as a communication in Lancet Diabetes and Endocrinology.
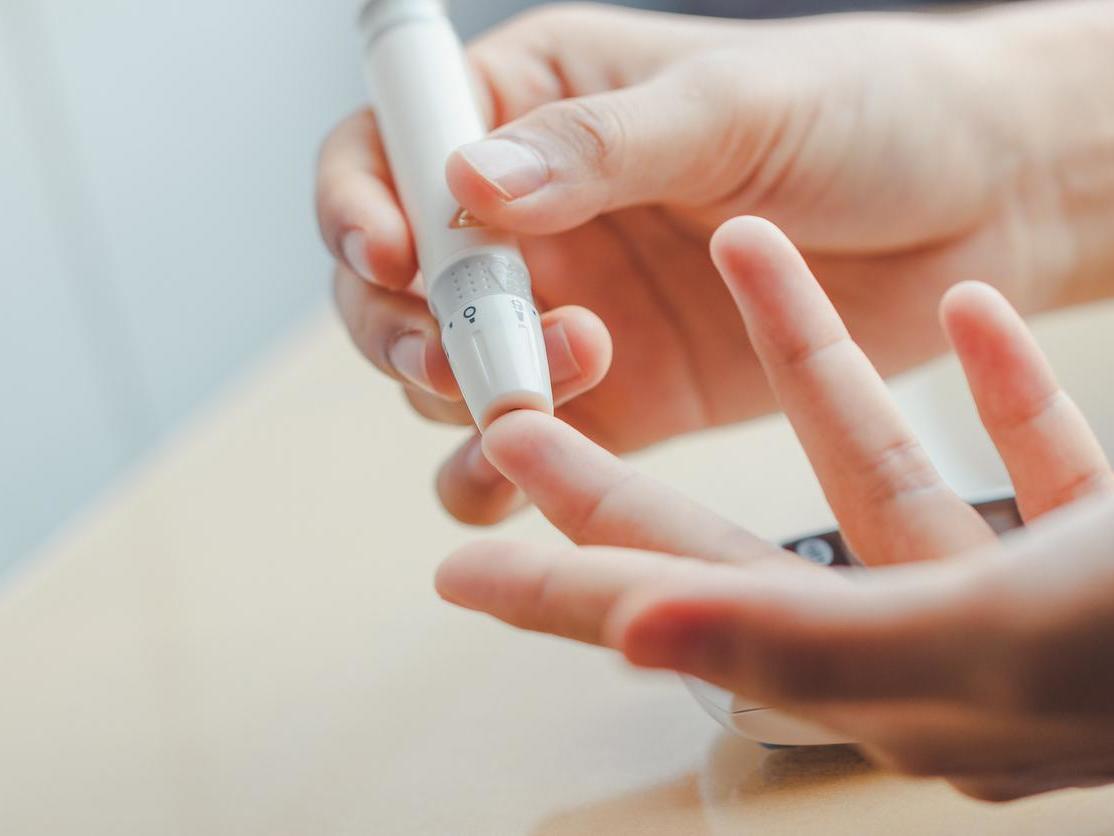
Children in the UK aged 3 to 13 years who did not have type 1 diabetes were invited to participate and provided fingerprint blood samples that were checked for antibodies previously found in symptomatic patients.
Children identified as potentially at risk of T1 diabetes are invited to have further blood tests or a glucose tolerance test.
A total of 17,283 tests were analyzed and more than 200 children were found to be at risk of developing T1D or to have markers in their blood that indicate T1D risk.
The next phase of the study, “Elsa 2,” will recruit more children across a wider age range, from 2 to 17 years old.
This part of the study will provide support to NHS clinics at 20 research centers across the UK for four years.
These clinics will help support and educate families of children who are found to be at risk for type 1 diabetes or have early-stage type 1 diabetes.
Staff will also be able to help children receive insulin treatments.
A new treatment – teplizumab – which can help delay the need for insulin treatment and was approved for use in the UK by the medicines regulator last year, could be available to some young people if it is approved by the NHS spending watchdog.
Lead researcher Parth Narendran, professor of diabetes medicine and honorary consultant physician at the University of Birmingham, told the Press Association: “This is a landmark study for the UK and shows for the first time that we can identify these people at an early stage and prevent emergency diagnosis.

“This gives families time to prepare.”
Speaking about the new clinic, he said: “Once people are diagnosed with early stage type 1 diabetes before they need insulin but we know they will continue to use insulin. They need support.
“So the idea is that they get regular support and advice about when to test their blood sugar and what symptoms to look out for, so they can start taking insulin as early and promptly as possible.”
He continued: “Once they are on insulin, they will naturally move into a normal Category 1 clinic with the same medical team. So it should be a seamless transition and a much gentler introduction to insulin therapy.”
In the future, teplizumab and other treatments in development may be available “so they don’t need insulin long-term and we get them in the early stages of type 1 diabetes and don’t need insulin,” he added.
“This is a huge improvement,” he said.
Up to 400,000 people in the UK have type 1 diabetes – around 8% of people with diabetes.
About a quarter of children with type 1 are diagnosed only during an emergency.

Professor Narendran says a new screening program could prevent children from “crash landing” with a diagnosis in future.
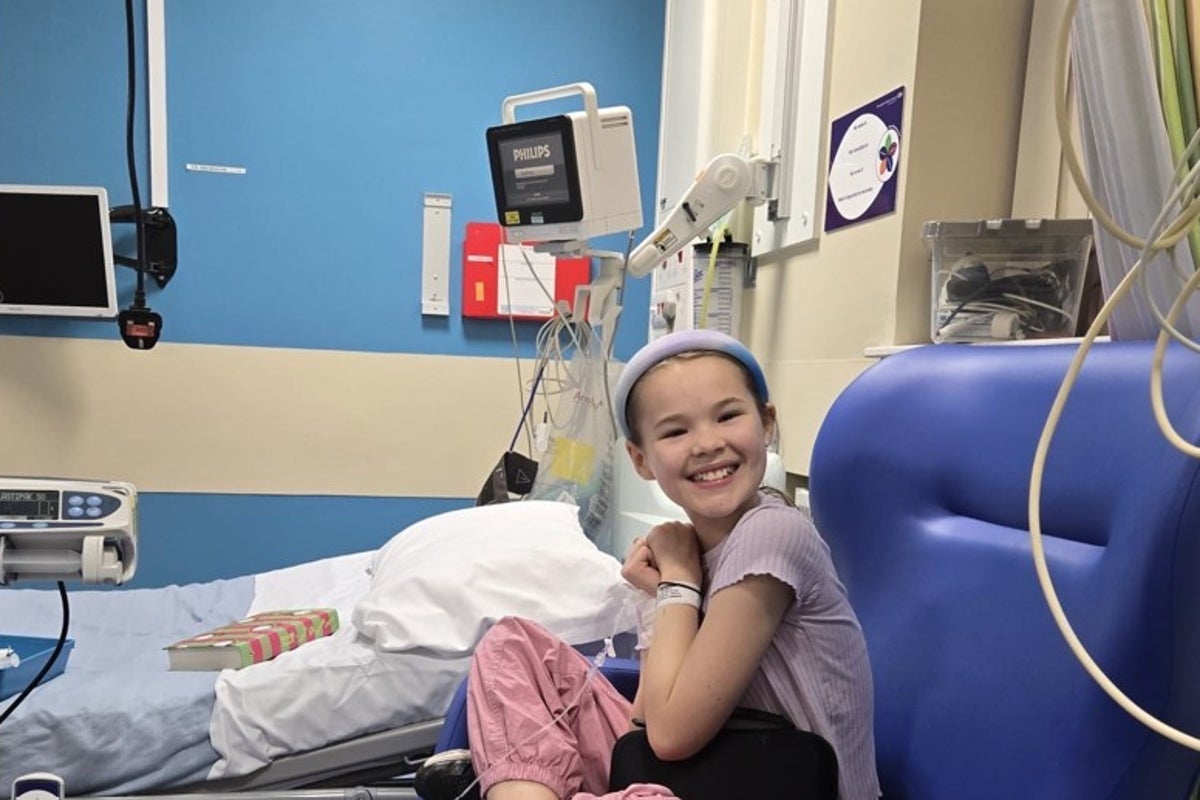
Amy Norman, 44, from the West Midlands, was diagnosed with type 1 diabetes when she was 13.
Elsa’s research revealed that her 11-year-old daughter Imogen was in the early stages of type 1 diabetes.
She is the second child in the UK to receive the immunotherapy drug teplizumab.
Ms Norman said: “Being involved in the Elsa study has helped prepare our family for the future in a way we never expected.”
“When I was diagnosed, I had no warning and ended up in hospital with diabetic ketoacidosis, which was really bad.
“Forewarned is prepared. She was always going to develop type 1 diabetes, but with Elsa we were able to slow down the process and be prepared – we knew what was coming, but we weren’t afraid.”
Dr Elizabeth Robertson, from the charity Diabetes UK, said: “For too many families, a child’s diagnosis of type 1 diabetes remains a feared emergency, but it doesn’t have to be this way.
“Elsa’s research is generating the evidence needed to make type 1 diabetes screening a reality for every home in the UK.”
Rachel Connor from Breakthrough T1D added: “This will rewrite the Type 1 diabetes story for thousands of families – we can provide time, choice and hope rather than a devastating emergency.
“By finding children at their earliest stages, we not only prepare families, but we open the door to treatments that can delay the need for insulin by years.”
Italy was the first country to launch a national screening program, and other countries are also looking to introduce screening for the disease.